Abstract
Background
Targeting of DNA synthesis by chemotherapy drugs is an effective strategy for the treatment of lymphoma. Disrupting these pathways with rationally designed small molecules is expected to improve potency but risks toxicity due to dependence of all dividing cells on DNA synthesis. The conversion of UTP to CTP, which is the rate limiting step in pyrimidine synthesis, is unusual in being catalysed by two homologous enzymes, CTPS1 and CTPS2. Studies of families with inherited CTPS1 deficiency indicate CTPS1 is essential for B and T cell proliferation whereas CTPS2 can compensate outside the blood system. This suggests that selective CTPS1 inhibition may deliver efficacy in lymphoma whilst avoiding systemic toxicity.
STP938 is a potent inhibitor of CTPS1 showing >1,300-fold selectivity over CTPS2. Efficacy against neoplastic human B and T cells has been demonstrated using in vitro and in vivo models. Importantly, STP938 induced death of neoplastic cells, establishing a cytotoxic mechanism of action. Pharmacokinetic, dose response and tumour growth inhibition data from in vivo models were used to generate a pharmacokinetic/pharmacodynamic model; this model predicts an efficacious dose in humans that is likely to be achievable by oral dosing, given the good oral bioavailability observed in preclinical species.
Study design and endpoints
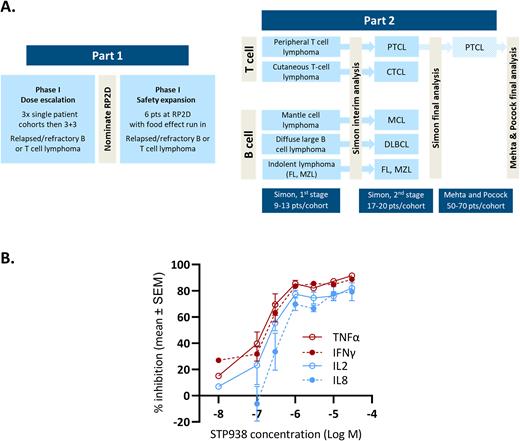
This study will employ a seamless dose escalation, dose expansion design (Figure A). STP938 will be given orally by continual twice daily dosing. The phase 1 dose escalation protocol will employ accelerated titration, enrolling three single patient cohorts (assuming no significant toxicity is observed) to reduce the number of patients receiving a potentially subtherapeutic dose. Cohort four will be at the predicted efficacious dose; this cohort onward will employ a standard 3+3 design. Intra-patient dose escalation will be permitted, to offer all patients the chance to receive an efficacious dose. The recommended phase 2 dose (RP2D) will be nominated based on safety, target engagement (see below) and early signs of efficacy. An additional six patients will be treated at the RP2D with a preliminary assessment of food effect. Key phase 1 endpoints are safety and tolerability. The key phase 2 endpoint is objective response rate.
Key eligibility criteria
The study will be open to patients aged 18 years or older who have received at least 2 prior lines of therapy and have no treatment options known to provide clinical benefit. The phase 1 study will recruit patients with B or T cell lymphoma; the phase 2 study will be limited to cohorts of patients with specific lymphoma subtypes, which may include peripheral T cell lymphoma, cutaneous T cell lymphoma, mantle cell lymphoma, indolent B cell lymphoma and diffuse large B cell lymphoma. Standard exclusion criteria apply; patients with ECOG performance score >2 or known CNS involvement by lymphoma will not be eligible.
Statistical analysis
The phase 2 study will follow a Simon two-stage design with an interim analysis for futility based on predefined, lymphoma subtype specific response rates. In the case of STP938 showing promising efficacy in one of the lymphoma subtypes, provision is included in the protocol to expand a cohort using an adaptive approach based on early efficacy signals (after Mehta and Pocock, 2011). This would permit expansion of a T cell lymphoma arm to generate enough statistical power for potential accelerated approval, should the efficacy signal be of sufficient strength and durability.
Pharmacodynamic and translational studies
Target engagement will be assessed indirectly by measuring the release of four cytokines (TNFα, IFNγ, IL2, IL8) from ex vivo stimulated patient T cells. In preclinical development, this assay demonstrated good dynamic range with maximal inhibition of T cell cytokine release at drug concentrations likely to be achievable in human subjects (Figure B). Exploratory studies will include pre- and on-treatment lymphoma biopsies to assess biomarkers of response and mechanism of action. Tumour genomics will be assessed by sequencing of circulating tumour DNA (ctDNA) prior to treatment and at disease progression using a bespoke genomic assay designed to elucidate biomarkers of response and understand mechanisms of resistance.
Current status
The study protocol has received FDA and MHRA approval. The phase 1 study (NCT05463263) will open to enrolment in the US and UK in August 2022.
Disclosures
Beer:Step Pharma: Current Employment. Higgins:Step Pharma: Current Employment. Asnagli:Step Pharma: Current Employment. Hoeben:Step Pharma: Consultancy. Parker:Step Pharma: Current Employment. Schwartz:Step Pharma: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal